Scripps Research rises to meet the challenge of the historic COVID-19 pandemic.
In early January, news outlets in the United States began reporting that a mysterious pneumonia-like illness was spreading through central China. The stories at first elicited only minor alarm but were a harbinger of a global health crisis like no one had seen in generations.
Soon, researchers in China identified the pathogen behind the illness as a coronavirus, from the same family of viruses as the one that caused SARS, or severe acute respiratory syndrome, a disease that first emerged in 2003. SARS’ newly discovered relative was dubbed COVID-19, a name that would soon be known and feared across the planet. Within nine months of those early news stories, there would be more than 30 million confirmed cases of COVID-19 and nearly 1 million deaths attributed to the disease worldwide.
As the outbreak began to spread in early 2020, Scripps Research scientists dropped everything to focus on the nascent viral pandemic. Among the institute’s scientists are leading experts on how viruses evolve, how they infect and sicken people, and how they spread through the human population. While some laboratories had to be temporarily shuttered to prevent an outbreak at the institute, many quickly pivoted to pursue essential COVID-19 research.
The institute’s immunologists and structural biologists focused on mapping the structure of viruses and studying how the immune system responds to it at the cellular and molecular level. Other labs analyzed the dynamics of how the virus emerged and how new technologies can be used to prevent, track and mitigate epidemics. At the beginning of the pandemic, Calibr, the drug discovery division of Scripps Research, had already built the most comprehensive drug repurposing library in the world. Known as ReFRAME, the collection contains more than 14,000 drugs, and soon would become a critical resource for scientists at Scripps Research and around the world looking for therapies against the mysterious and deadly new coronavirus.
In the following pages, you will find stories from the first nine months of Scripps Research’s efforts to help decipher and mitigate COVID-19. Our research continues.
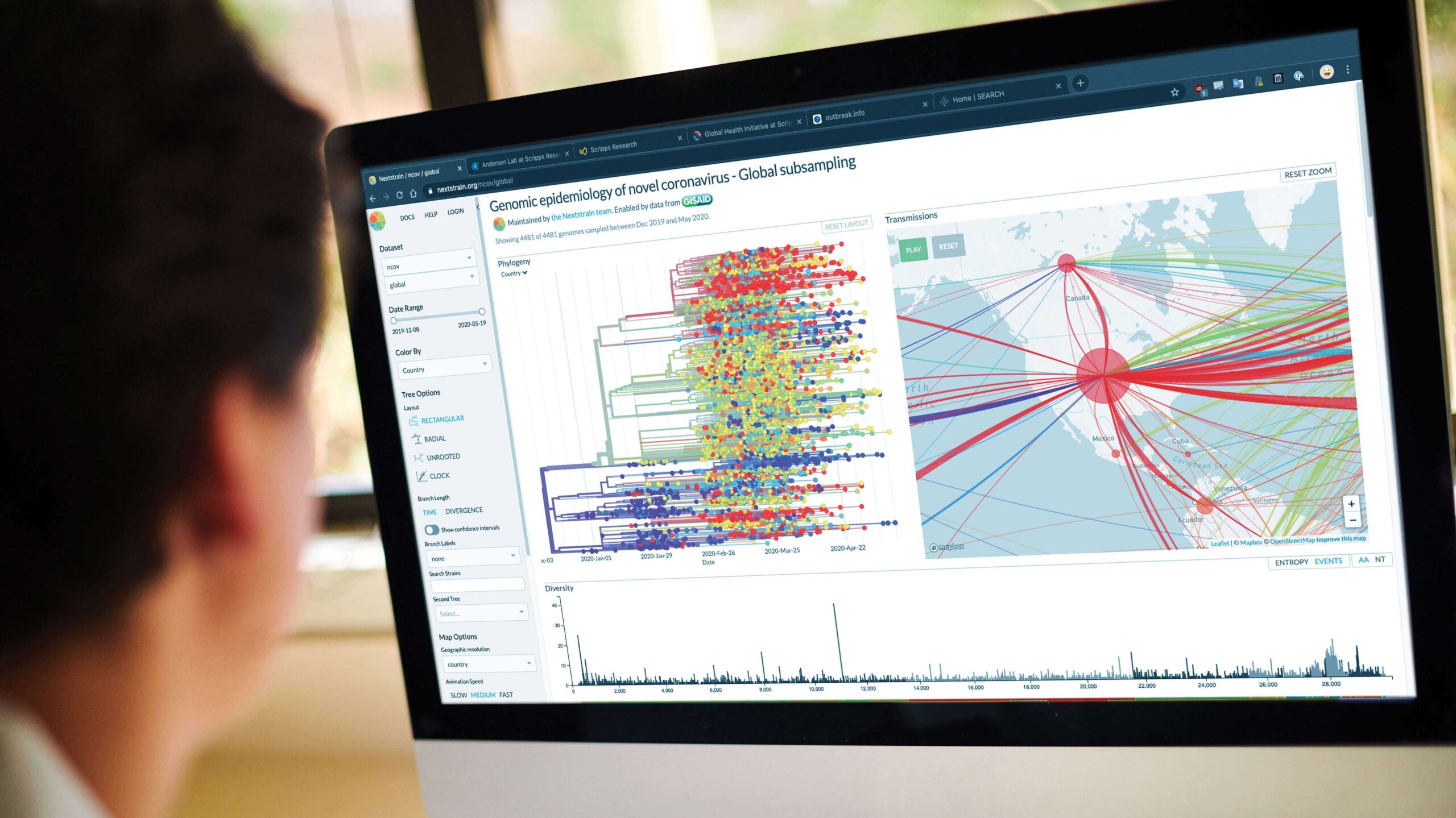
Tracing the virus, from its origin to its global spread
Could nature really invent a virus that spreads so easily around the world, enacting such a massive toll on human life—not to mention the economy?
Yes, it can. And it did.
As misinformation and conspiracy theories about the origin of the novel coronavirus abounded, Kristian Andersen, PhD, emerged as a beacon of science and reason.
Soon after the COVID-19 outbreak started in China, Andersen and his team of genomic epidemiologists began working with collaborators around the globe to figure out how the SARS-CoV-2 virus emerged.

“Most theories considering a link to the lab are conspiracy theories. They are exceptionally disruptive to the efforts of getting COVID-19 under control and result in unnecessary human suffering.”
—Kristian Andersen, on the dangers of misinformation: July 2020
In a widely cited study in Nature Medicine—which has gained more online attention than any study ever published, according to the research tracking service Altmetric—Andersen concluded that the virus undoubtedly evolved in nature and was not deliberately engineered.
By analyzing the virus’s genomic data and molecular structure, he and his colleagues found stark similarities to coronaviruses in bats and pangolins, while revealing subtle flaws indicative of natural selection.
Questions remain about exactly how the virus jumped from animals to humans, but Andersen has made clear that conspiracy theories of lab-made bioweapons must be put to rest: “Those hypotheses are not consistent with the data we see, and they don’t explain how SARS-CoV-2 emerged,” says Andersen, professor of Immunology and Microbiology.
Next, the team drew from its experiences combating recent epidemics of Ebola, Lassa and Zika viruses to understand how the coronavirus is spreading. One major project is decidedly local: Working with several other institutes and health organizations in San Diego, Andersen launched a large-scale COVID-19 screening study called SEARCH, with an initial focus on healthcare workers, firefighters and other first responders.
They hope to gather more complete information about how the virus is moving through the community—knowledge that will help public health officials gain the upper hand on the pandemic.
“When does this end? Until we have a vaccine, it doesn’t,” Andersen says. “What we need are long-term plans for how to live with the virus. We need better early detection, better contact tracing and consistent isolation for those who are infected. That is the only way.”

Repurposing existing drugs for a new threat
When the COVID-19 pandemic began to make headlines out of China, Malina Bakowski, PhD, was focused on another part of Asia. She was working with researchers in Thailand, Cambodia and at University of Georgia to find drugs that could be repurposed against malaria. They were screening for anti-malaria drug candidates using ReFRAME, a drug repurposing library compiled by researchers at Scripps Research’s drug discovery division, Calibr.
“When it became evident that we were dealing with a major pandemic, we shifted gears to focus on COVID-19,” says Bakowski, a principal investigator at Calibr.
Led by Peter Schultz, PhD, the president and CEO of Scripps Research, Calibr established the ReFRAME collection in 2018 with support from the Bill & Melinda Gates Foundation to tackle areas of urgent unmet medical need, especially neglected tropical diseases. The collection has since grown to comprise over 14,000 compounds, including drugs that are already being repurposed for a number of diseases.
As the pandemic spread early in the year, Arnab Chatterjee, PhD, vice president of Medicinal Chemistry at Calibr, led efforts to team up with labs at Scripps Research and other institutions in the United States, Europe and Asia to search ReFRAME for individual drugs or combinations that may be effective in treating people exposed to SARS-CoV-2, the coronavirus that causes COVID-19.
“Early in the COVID-19 pandemic, we saw that ReFRAME could be leveraged to screen for hits against SARS-CoV-2,” says Chatterjee. “Since then, we have launched more than 20 scientific collaborations to have a near-term impact on COVID-19.”

A collaboration with Dennis Burton, PhD, chair of the Department of Immunology and Microbiology at Scripps Research, and Thomas Rogers, MD, PhD, an adjunct assistant professor at Scripps Research and assistant professor of Medicine at UC San Diego, has proven particularly fruitful. Working in a specialized biosafety level 3 laboratory, the scientists treated human cells infected with SARS-CoV-2 with each of 12,000 drugs from ReFRAME. After 24 hours, they measured the level of viral infection in the cells to determine if the drugs prevented the virus from replicating. In some cases, they applied two drugs at a time to see if the compounds would work together against the virus.
The study identified more than two dozen existing drugs or drug candidates with antiviral activity against the novel coronavirus. Several of the drugs—halofantrine, amiodarone, nelfinavir, simeprevir, manidipine, ozanimod and osimertinib— are already FDA approved for use in humans, and 19 others are in various stages of the drug development process.
A disease as confounding as COVID-19 is unlikely to be treated with a single drug. Early on, the team led by Schultz and Chatterjee focused on the evaluation of drug combinations, inclined to find ways to enhance the activity of drugs—such as Gilead’s remdesivir—already being tested in humans. The researchers found 24 drugs that had an additive effect when administered with remdesivir, the antiviral produced by the pharmaceutical company Gilead, that is currently being tested in human clinical trials against COVID-19. An additive effect means that the drugs were both active against the virus when applied together.
Based on the results of cell culture screens, the researchers are now working to test the best-performing drug candidates in animal models to determine which are most likely to work in human patients. Those studies will pave the way to human clinical trials.
“The results from the cellular assays are very promising and the need for medical remedies to address COVID-19 is incredibly urgent. It is critical we proceed with the utmost rigor to determine what is safe and effective, as diligence is the most expedient path to finding new therapies that will make a difference for patients.”
—Peter Schultz, PhD
President and CEO, Scripps Research
Mining for the most powerful COVID-19 antibodies
Circulating in the blood of recovered COVID-19 patients are some of the best weapons against the virus: antibodies, which the immune system produces upon an encounter with an invader.
However, not all antibodies are particularly effective at taking down SARS-CoV-2, the virus that causes COVID-19. It’s the job of immunologist Dennis Burton, PhD, to find the ones that are.
“The strongest antibodies could be fashioned into drugs and preventative medicines,” Burton says. “Or, they can be used to guide the development of a vaccine that will help put an end to the pandemic.”
In early February, Burton’s lab was still focused almost entirely on HIV—a virus known to be extremely challenging due, in part, to its fast mutation rate. For years, his team has been working to find so-called “broadly neutralizing” antibodies that protect against most or all strains of HIV.
But as soon as the imminent threat of COVID-19 became clear, Burton made a shift that proved to be fruitful. Working in collaboration with the vaccine nonprofit IAVI and the University of California San Diego School of Medicine, Burton’s team put out a call for blood samples from recovered COVID-19 patients. The response was overwhelming.
Using samples from patients with the most potent immune responses, the team isolated more than 1,000 antibodies and evaluated how each one responded to COVID-19 in a test tube and in animals. A handful prevailed in both settings.
The study—co-led by Thomas Rogers, MD, PhD, an adjunct assistant professor in the Department of Immunology and Microbiology at Scripps Research, and assistant professor of medicine at UC San Diego—appeared in the journal Science.
Though Burton’s lab has resumed much of its HIV work, he is looking to team up with a pharmaceutical partner to produce the antibodies in greater quantities so they can be explored in the clinic. He also would like to identify broadly neutralizing antibodies that protect against all coronaviruses, including those that haven’t yet emerged.
Burton notes that COVID-19 is the third dangerous coronavirus-driven disease to emerge in the past 20 years—following MERS, discovered in 2012, and SARS, in 2003.

“A new coronavirus will almost certainly come again in the future,” says Burton, co-chair of the Department of Immunology and Microbiology. “When it does, we want to be better prepared. That’s why it’s imperative to continue working toward a vaccine or treatment that can take down all coronaviruses.”
—Dennis Burton, PhD
Finding the enemy’s weakness
Ian Wilson, DPhil, had long feared a new influenza virus would become the next global pandemic. “I’ve worried that in the worst case, it might be like 1918,” he says.
For this reason, his structural biology lab has keenly focused on the flu for nearly 40 years.
By early 2020, it became clear that Wilson’s concern about a new pandemic was not unfounded. “However, it turned out to be a coronavirus, not influenza,” says Wilson, chairman of Scripps Research’s Department of Integrative Structural and Computational Biology.
Fortunately, his lab’s groundbreaking work on influenza—in addition to other viruses, such as HIV and hepatitis C—gave him a stockpile of expertise and the right technologies to begin immediately examining the SARS-CoV-2 virus for weak spots that could be exploited by a vaccine or treatment.
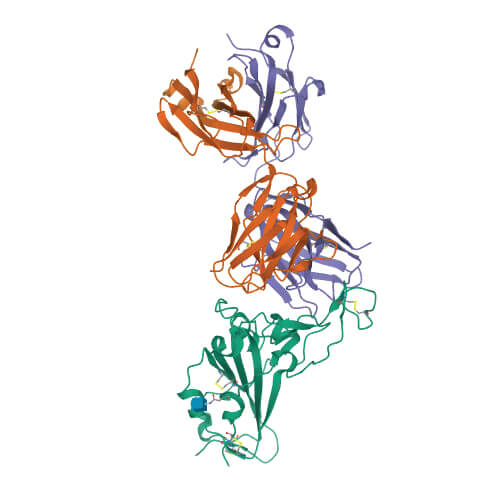
Using sophisticated imaging equipment, Wilson’s team quickly pinpointed a potential Achilles’ heel. Wilson and others in his lab—including Meng Yuan, PhD, and Nicholas Wu, PhD—found that an antibody produced by the body in response to a previous coronavirus infection, SARS, attached to a nearly identical spot on the new coronavirus. This suggested a functionally important and vulnerable site for this family of coronaviruses.
“The knowledge of sites like this on the virus can help structure-based design of vaccines and therapeutics against SARS-CoV-2, and these could also possibly protect against other coronaviruses— including those that may emerge in the future,” Wilson says.
The discoveries kept coming, helped by the fact that more antibodies to COVID-19 became available to researchers after the first local patients recovered. In July, after reviewing nearly 300 of these antibodies, Wilson worked with Dennis Burton’s lab to identify a common feature among the most effective antibodies: they all stemmed from the same genetic source. The findings appeared in the journal Science.
It was good news, as this type of antibody is usually present at low levels in the blood of healthy people. It suggested that boosting these antibodies, as a vaccine could do, should be able to prevent or treat the disease.
Although several potential vaccines are already in clinical trials, scientists don’t yet have a full understanding of the molecular features that define a successful antibody response. In the new study, the scientists took a big step toward filling that knowledge gap.

“Amidst all of this, it’s been gratifying to observe the overwhelming response from the community and from those who have recovered from COVID-19,” Wilson says. “Their efforts, from providing blood samples to supporting ongoing basic research, is making a real difference as we all work to create vaccines and therapeutics.”
—Ian Wilson, DPhil
Mapping the course for coronavirus vaccines

“This is the greatest science experiment in vaccinology that’s ever been done. It’s literally testing all the different technologies, and it’s going to be cool to see how this all shakes out.”
—Andrew Ward on global efforts to create the first-ever approved RNA vaccines.
Andrew Ward, PhD, was studying coronaviruses long before they became a household name.
More than three years ago, using some of the most advanced electron microscopy equipment in the world, his lab revealed the very first structure of a human coronavirus spike protein. The protein came from the HKU1 coronavirus, which causes seasonal colds.
His lab then went on to do the same for more deadly coronaviruses: SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome).
“The reason we initially decided to work on coronaviruses was the expectation that they could cause a pandemic,” says Ward, professor of Integrative Structural and Computational Biology. “Though I can’t say I expected anything like this to happen, or to happen so quickly.”
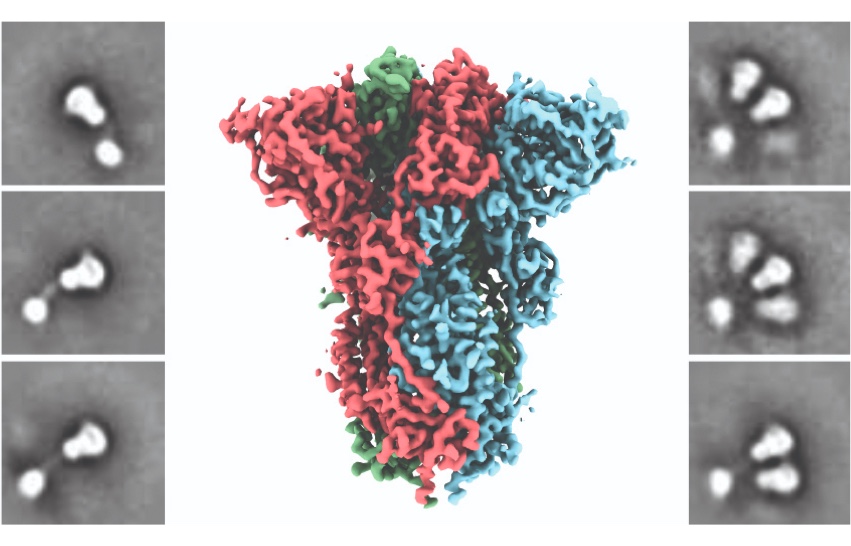
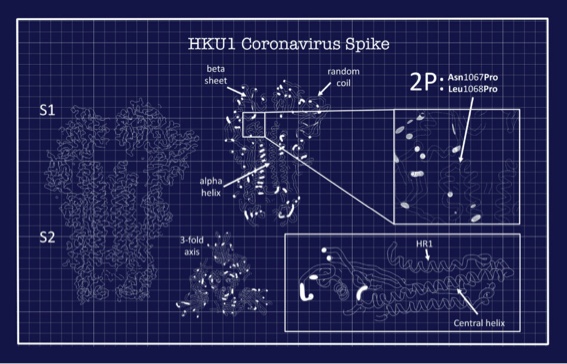
Coronaviruses use their spike proteins to invade human cells. Understanding the protein’s shape and seeing how spikes interact with protective antibodies is a critical step in designing a vaccine, Ward explains. The structures he creates using advanced microscopy techniques are, in essence, atomic-level blueprints from which vaccines can be designed.
Several years ago, these blueprints enabled Ward and his colleagues to engineer a way to stabilize the spike proteins so that they could be produced at large scale and keep their integrity in a vaccine. This innovation is used in several coronavirus vaccines now in development, including the potential COVID-19 vaccines being advanced by the pharmaceutical companies Moderna and Novavax. By exposing the immune system to this engineered spike via a vaccination, the body recognizes it as foreign and makes neutralizing antibodies that can quickly fight back upon real-life exposure to the coronavirus. “It’s an illustration of how basic research and structural biology directly contribute to creating vaccines,” Ward says.
In spring of 2020, as the first genetic sequence of SARS-CoV-2 emerged, Ward and his team instantly turned their focus to the newest member of the coronavirus family. “It was literally plug-and-play,” he says. “We already had the expertise and processes in place. That’s why everything moved so fast.”
The group is now working with public and private vaccine makers on new efforts to defeat COVID-19. As vaccines move forward in human trials, Ward says his team is transitioning from examining immune system interactions with SARS-CoV-2 in animals to those in actual patients.


A vaccine prototype in under two months
Scripps Research scientist Jiang Zhu, PhD, applied his nanoparticle vaccine technology—which has already been used to develop experimental vaccines for HIV, hepatitis C, Ebola and respiratory syncytial virus—to create a potential vaccine for the virus that causes COVID-19.
Zhu is cofounder of the 2018 Scripps Research spinoff company Ufovax, which is built around his development of a vaccine platform that can be rapidly applied to a wide range of diseases. The vaccines rely on miniscule viral particles that induce potent immune responses, and they’re designed to be easily manufactured on a large scale.
By mid-March, Zhu and his team had produced a prototype of a coronavirus vaccine, featuring SARS-CoV-2 protein spikes protruding from a nanoparticle “scaffold.” Zhu called it a “crucial first step” in efforts to develop an effective vaccine against COVID-19. He’s now running advanced tests of the prototype to gauge immune response in animals, and eventually in people.

Finding the mutation that enables coronavirus to infect with ease
They say the little things make a marriage work. If so, Hyeryun Choe, PhD, and Michael Farzan, PhD, together more than 20 years, seem ideally matched, discussing nanoscale virus mutations late into the evening, like most people discuss food, friends or weather.
“Viruses with more functional spikes on the surface would be more infectious. And there are very clear differences between the two viruses in the experiment. Those differences just popped out.”
— Michael Farzan on his and Hyeryun Choe’s discovery of a mutation that appears to make the coronavirus more infectious.
The virus that causes COVID-19 is only about 130 nanometers in size. For scale, if the coronavirus were a car, taking a journey across the width of a human hair would be akin to driving from San Diego to Reno. Although this makes the virus invisible in light microscopes, Farzan and Choe speak of each tiny mutation as if it were a person they know on sight.
“There are very clear differences between George and David,” Farzan says, using the names as shorthand with a pair of The New York Times reporters interviewing them on Zoom. David stands for aspartic acid at position D614 in the virus spike. George is glycine at the same position, D614G.
“George is really more infectious,” Choe agrees, leaning into her computer camera at the couple’s condo office. “George has about four times more spike proteins.”
Both Choe and Farzan are virologists. Their work has braided across their distinct interests: Farzan homes in on chemical structures and antibodies; Choe thinks deeply about viral entry into cells. They’ve gone different directions with their science through the years, but now they’re pooling their talents to address the pandemic SARS-CoV-2 coronavirus, cause of COVID-19.
They’ve studied coronaviruses since the first SARS outbreak nearly 20 years ago—working together, they co-discovered the receptor that SARS uses to infect cells. The new coronavirus uses the same receptor, called ACE2. With their deep knowledge, they’ve never been so in-demand, or found their scientific efforts so intertwined.
“Mike is the creative element. He’s got 100 ideas a day,” Choe says. “Once in a while, a good one.”
“Hyeryun is the analytical one. If something passes her test, we know we are on to something,” Farzan says, smiling. “Most things don’t.”
The two met at Harvard in the late 1990s, when Farzan was pursuing his doctorate and Choe was a postdoctoral researcher helping train him.
“We fought constantly,” Farzan laughs.
Over what? “Everything,” Choe answers. “Bench space, hypotheses, authorship, how to do experiments.”
“Especially bench space. I was consigned to a desk until my skills improved,” Farzan adds.
The decision to move to Scripps Research in Florida was an easy one for the couple in 2012.
Farzan had grown up in Boca Raton, where his dad worked on the IBM campus. He loved the idea of returning to the Sunshine State. Plus, they wanted to actually help make new vaccines and medicines, to see their discoveries reach the clinic and have an impact. Scripps Research is a place where that can easily happen.
In addition to co-chairing the Department of Immunology and Microbiology, Farzan now nurtures two start-up companies focused on advancing his and Choe’s inventions. Emmune, in Juno Beach, uses gene therapy techniques to make antibody therapies for viral diseases including HIV and COVID-19. Bliss, in Boston, is advancing a COVID-19 vaccine prototype.
On arriving in Florida, Choe set up her lab in a different building. Before the pandemic, her research program focused on Dengue and Zika viruses. Now it’s mostly coronavirus again.
For all the stress and pressure of staring down a virus that has killed hundreds of thousands of people and hobbled the economy, the couple’s optimism is as obvious as the spikes on George.
Farzan is quite confident that scientists will iterate a broadly effective vaccine in record time. Choe feels certain that the vaccine the two of them developed and tested is the best possible candidate. And, good news, it appears to work on both George and David.
“It’s still an amazing journey,” Choe says. “I think we feel lucky that our work might be useful.”

Detecting infection earlier with fitness wearables
The wrist-worn fitness trackers that motivate so many Americans to stay active are serving another key role during the pandemic: detecting viral illnesses, including COVID-19.
Jennifer Radin, PhD, an epidemiologist at Scripps Research Translational Institute, is leading a nationwide study to determine whether fitness trackers can predict outbreaks faster than what’s currently possible.

“When people get an infection, their resting heart rate tends to increase and their daily activities will change, as will sleep patterns.”
—Jennifer Radin on how smartwatches can pick up on illness even before symptoms emerge.
“Early detection is critical for effective public health response to infectious disease outbreaks,” Radin says. “We believe we can do this by leveraging the rich health data that’s already being collected by the millions of Americans who regularly use wearable devices.”
The study, called DETECT, kicked off in March, just as offices and schools were shutting down. By early summer, well over 35,000 people had enrolled across the United States, consenting to share their de-identified data via an app called MyDataHelps that communicates with their tracking device.
The data is then analyzed for individual changes in sleep patterns, heart rates and activity levels—all of which are known to stray from their norm during an infection. Participants also log any symptoms they’re experiencing or test results they receive.
By mid-June, 54 participants had tested positive for the virus, 279 reported testing negative and thousands noted having one or more symptoms.
Preliminary results of the study showed that even with the small initial sample size, researchers significantly improved the distinction between symptomatic individuals with and without a diagnosis of COVID-19, compared with an evaluation of symptoms alone.
“Common screening practices for COVID-19 typically include temperature measurements and surveys focused on symptoms and travel,” Radin says. “However, these methods are likely to miss pre-symptomatic or asymptomatic cases, and that can foster further disease spread.”
The DETECT study is still open for enrollment; Radin hopes to sign up more than 100,000 participants. Learn more at detectstudy.org.

“In the setting of infectious disease, you can test negative one day and positive the next. A wearable health tracker, on the other hand, provides a continuous, unobtrusive, real-time assessment so you can see a problem emerging even before symptoms.”
—Eric Topol, MD
Director and Founder, Scripps Research Translational Institute